1. Place the cadaver in the left lateral decubitus position.
The position is important in the case of ruminants, as the volume of the rumen is large and can make examination of the abdominal cavity difficult if it needs to be accessed.
2. Open the stifle joint to examine the synovial capsules.
When an incision is made into a healthy joint, the presence of synovial fluid is minimal. If synovitis is suspected, a sample of synovial fluid should be taken to investigate the possible presence of Mycoplasma bovis or Histophilus somni, respiratory pathogens that also tend to colonize these tissues.
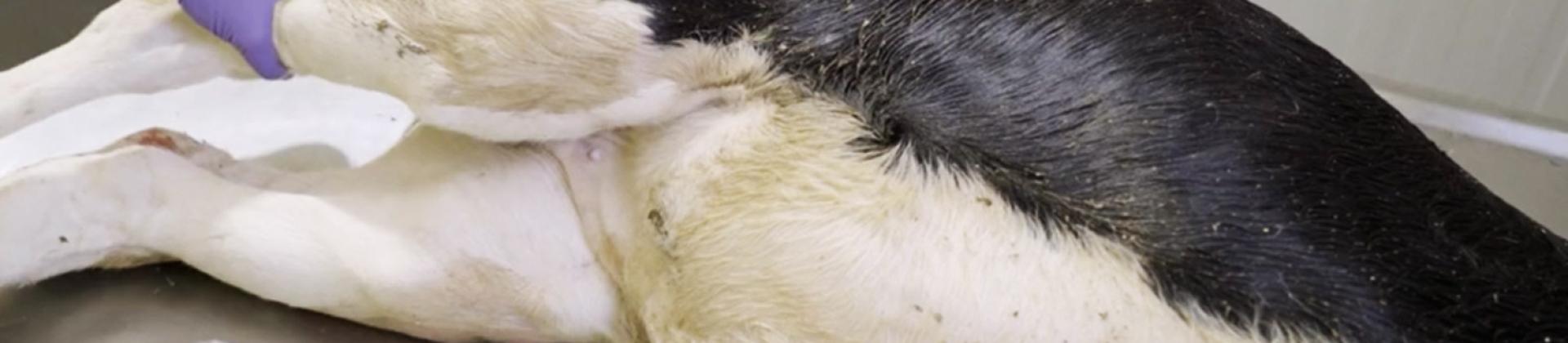
3. Dissect the right foreleg.
Make an incision in the axilla, the leg muscle attachment site, at the same time as the limb is abducted, until the thorax can be easily accessed.
4. Expose the trachea as far as the pharynx.
Detach the trachea completely, including the pharynx, so that the whole respiratory tract can be subsequently removed.
5. Open up the ribs.
Make long incisions in the intercostal spaces, ensuring that the lung is not damaged. Cut along the line of the sternum, which will allow the thoracic cavity to be opened. In older animals, an
axe may be needed to cut along the line of the sternum. Breaking the ribs manually, open the cavity until the lung is exposed.
At this point, we are able to observe the appearance of the lung and the visceral and parietal pleura. We will also be able to see if there is any abnormal fluid in the cavity.
6. Remove the respiratory tract.
Completely remove the respiratory tract, from the pharynx to the lungs. Place it on a clean surface which allows the organs to be manipulated easily.
7. Inspect the lung tissue.
Use scissors to make several cuts deep into the lung parenchyma, along the line that separates the healthy from the unhealthy tissue. Repeat this in the affected area at a distance of 1 or 2 cm from the previous incisions. Take samples by scraping vigorously with the swabs inside each incision in order to detect the presence of viruses and bacteria. Small samples of affected tissue may be cut out and placed in a vial to be sent to the laboratory.
8. Check for the presence of abscesses or other lesions in the pharynx.
9. Open the trachea as far as the bifurcation.
Note the presence of lesions and collect samples by rubbing vigorously with a swab to look for the presence of viruses, such as the Bovine Herpesvirus I (IBR), which has a great affinity for this organ.
10. Cut the myocardium into strips.
Make several incisions to reveal the presence of infarcts, abscesses or myocarditis, indicators of possible Histophilus somni infection. If this is suspected, samples can be taken with a swab for PCR.